
Tantalizingly close, life might be living on Mars as we speak based on what Curiosity has discovered.
Any strong carbon signal is intriguing when you’re hunting for life. It’s a common element in all the forms of life we know of. But there are different types of carbon, and carbon can become concentrated in the environment for other reasons. It doesn’t automatically mean life is involved in carbon signatures.
Carbon atoms always have six protons, but the neutron count can vary. Carbon atoms with different numbers of neutrons are called isotopes. Three carbon isotopes occur naturally: C12 and C13, which are stable, and C14, a radionuclide. C12 has six neutrons, C13 has seven neutrons, and C14 has eight neutrons.
When it comes to carbon isotopes, life prefers C12. They use it in photosynthesis or to metabolize food. The reason is relatively simple. C12 has one fewer neutron than C13, which means that when it bonds with other atoms into molecules, it makes fewer connections than C13 does in the same situation. Life is essentially lazy, and it will always seek the easiest way to do things. C12 is easier to use because it forms fewer bonds than C13. It’s easier to get at than C13, and life never takes the hard way when an easier way is available.
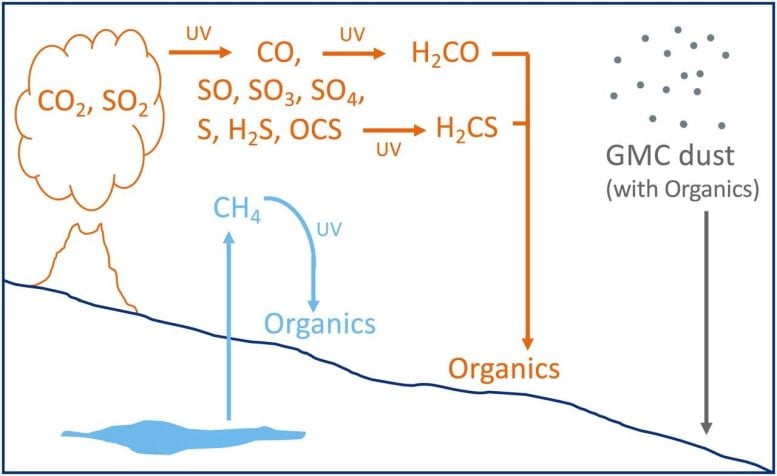
This figure from the study shows the three hypotheses that could explain the carbon signature. The blue shows biologically produced methane from the Martian interior, creating the deposition of 13C-depleted organic material after photolysis. The orange shows photochemical reactions via UV light that can result in various atmospheric products, some of which would be deposited as organic material with easily-broken chemical bonds. The grey shows the molecular cloud hypothesis. Credit: House et al. 2022.
The team behind Curiosity’s SAM looked at 24 rock samples with this process and recently discovered something noteworthy. Six of the samples showed elevated ratios of C12 to C13. Compared to an Earth-based reference standard for C12/C13 ratios, the samples from these six sites contained greater than 70 parts per thousand more C12. On Earth, 98.93% of the carbon is C12 Earth, and C13 forms the remaining 1.07%.
The upshot ,,,
“On Earth, processes that would produce the carbon signal we’re detecting on Mars are biological,” House added. “We have to understand whether the same explanation works for Mars or if there are other explanations because Mars is very different.”

NASA’s Curiosity rover raised its robotic arm with the drill pointed skyward while exploring Vera Rubin Ridge at the base of Mount Sharp inside Gale Crater – backdropped by distant crater rim. This Navcam camera mosaic was stitched from raw images taken on Sol 1833, October 2, 2017, and colorized. Credit: NASA/JPL/Ken Kremer/kenkremer.com/Marco Di Lorenzo.
Tantalizingly close indeed.


No comments:
Post a Comment